Education as a Business Opportunity
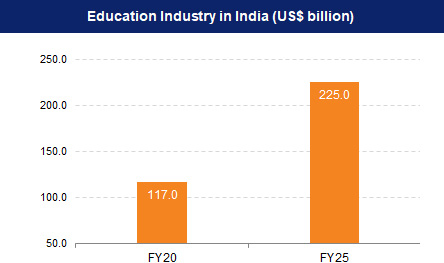
India has the world’s largest population in the age bracket of 5-24 years of about 500 million people, which provides a great growth opportunity for the education sector. The education sector in India was estimated to be worth US$ 117 billion in FY20 and is expected to reach US$ 225 billion by FY25.
India has over 250 million school-going students, more than any other country.
Number of colleges in India reached 42,343 in FY20. As of May 17, 2021, the number of universities in India stood at 981.
In 2021-22, as of February 2022, there were 8,997 total AICTE approved institutes in India. Out of these 8,997 institutes, there were 3,627 undergraduate, 4,790 postgraduate and 3,994 diploma institutes.
India had 38.5 million students enrolled in higher education in 2019-20, with 19.6 million male and 18.9 million female students.
In FY20, Gross Enrolment Ratio (GER) in Indian higher education was 27.1%.
According to UNESCO’s ‘State of the Education Report for India 2021’, the Pupil Teacher Ratio (PTR) at senior secondary schools is 47:1 as against 26:1 of the overall school system.
The Indian edtech market size is expected to reach US$ 30 billion by 2031, from US$ 700-800 million in 2021.
According to KPMG, India has also become the second largest market for E-learning after the US.
The online education market in India is expected to grow by US$ 2.28 billion during 2021-2025, growing at a CAGR of almost 20%. The market grew by 19.02% in India in 2021.
Clinical Research as a Business Opportunity:
The clinical trials market is projected to reach USD 52.0 billion by 2026 from USD 38.7 billion in 2021, at a CAGR of 6.1% during the forecast period. Factors such as increased R&D expenditure, growing demand for outsourcing of R&D activities, and the rising number of clinical trials for various diseases worldwide are driving the growth of the global market.
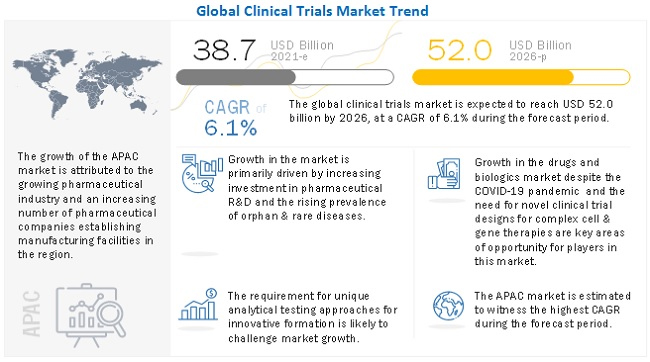
Covid-19 Impact on the Global Clinical Trials Market
A number of established pharmaceutical and biopharmaceutical companies have scaled up R&D and manufacturing efforts to develop and distribute test kits, vaccines, and drugs against the SARS-CoV-2 virus. To expedite the R&D process, many pharmaceutical and biotechnology companies teamed up with clinical trial service providers through long-term agreements, partnerships, and collaborations across the globe. The pandemic phase posed several challenges for professionals operating in the market space and trial sponsors. This, in turn, has created a dynamic change in the market, particularly in terms of clinical trial supply and logistics.
The onset of SARS-CoV-2 infection compelled various biotechnology entities to rapidly develop and commercialize vaccines against COVID-19. As reported by the WHO, the vaccine candidate landscape for COVID-19 witnessed a significant boost in the second half of 2020. The WHO reported that more than 80 vaccines are presently in the clinical trial phase. This indicates that the demand for COVID-19 vaccine trials is expected to remain high in the next 2 to 3 years. Additionally, due to COVID-19, the demand for ready-to-plug-in infrastructure to expedite timelines of clinical trials amidst the restrictions imposed by the pandemic has increased.
This has specifically increased the demand for decentralized services. Clinical research entities have also developed dedicated services for COVID-19 research. In 2020, a number of new service launches and agreements, partnerships, and collaborations with different pharmaceutical companies and academic institutes for the research & development of COVID-19 vaccines, drugs and diagnostics were recorded across the globe.
While there has been a huge growth in the seats for BDS, Pharmacy, Nursing, BAMS, BHMS, Biotechnology, Physiotherapy and other life sciences courses the job opportunities are very few and post-graduation such as MDS are not only very expensive but the return on time and money invested remains uncertain.
To meet this demand for Clinical Research professionals; Anovus has taken the initiative towards India’s first modular M.Sc (and Diploma) programme from UGC approved University, Desh Bhagat University.
So here is a good business opportunity for you while you also help create young professionals and build bright careers.
Our Strengths:
- Started in 2009, Anovus is one of the first in the field of Clinica research & Pharmacovigilance in the country.
- First Institute offering M.Sc. In Clinical Research from UGC approved, University, Desh Bhagat University, Mandi Gobindgarh.
- ANOVUS equipped with high quality courseware prepared and reviewed by senior Industry - academia professionals.
- Entire course design, curriculum and industry connect from the top notch people on Anovus Advisory Board
- Globally Validated Curriculum and teaching methodologies
- Support to our Strategic Partner in all stages of establishment: From day one to commencement and thereafter
- Support of a huge alumni base working in virtually every company of repute in the Clinical Research & Pharmacovigilance domain in the country.
Added Advantage:
When you take this initiative, you will acquire status of Business partner of Anovus Institute of Clinical Research with a potential to also offer courses as a Centre of Excellence of DBU.
Becoming an Anovus Franchisee:
There will be an MOU signed with ANOVUS.
As a result of this MoU with us Anovus will:
- Allow use of the ANOVUS branding
- Provide lectures and Presentations
- Help with Faculty selection and also training, if required
- Provide Teaching Schedule & Methodology
- Provide formats for practicals
- Industry connect
- Guidance on examinations
- Web presence & support through website and social media
- Printed collaterals formats for Prospectus, Brochures, Flyers, and Promotional folders, E-mailers, Posters, Advertising Templates, Signage and Media Publicity.
Who can be Franchisee partners?
As quality is the Top most priority we select our knowledge partners with utmost care. Some parameters that Anovus looks forward in its strategic associates are as follows:
- A strong entrepreneurial spirit and zeal to succeed.
- Long term Interest to prosper in the field.
- Commitment to healthcare education as business.
- Adequate resources
- Qualification in Clinical Research, Medicine or Pharmacology or experience in Clinical Research, though not essential, will be an added advantage
To grab this opportunity in your area contact:
Dr. Dharinder Tayal
Email Id:dharinder.tayal@anovus.net
+91-9814000720






